by Michael Howell
While people can get sick and die within days or weeks from exposure to certain highly contagious and highly lethal viruses, the work being done to combat such outbreaks moves at a glacial pace, it seems. But that is not without good reasons, the main one being an ancient imperative enshrined in medical and therapeutic traditions and first put in writing by the Greek physician/philosopher Hippocrates: “First of all, do no harm.” If you are going to produce a vaccine to fight off a disease, this means, be sure it works before you go out jabbing people in the arm. It is this ethical imperative, more than anything else it seems, that tends to keep vaccine production and use moving in slow motion. But you can add to that the extremely high financial costs and the often nightmarish political and technical issues that can slow or stall distribution as well. It all takes time.
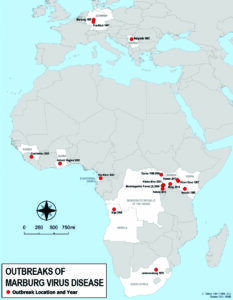
The pace can be especially frustrating for those looking for some relief on the battleground of hemorrhagic viral outbreaks around the world. A vaccine (ERVEBO) was developed to provide immunity to the Ebola Virus, one of the most severe hemorrhagic viruses, in 2005, but it was not until November 2019 that the vaccine was licensed for use in the human population. A vaccine has also been developed to treat other filoviruses such as the Sudan Virus (also an Ebolavirus) and the Marburg Virus.
Marburg belongs to the same family of viruses as Ebola, so the diseases can look similar. Both are characterized by viral hemorrhagic fever, a condition that can cause internal bleeding and damage multiple organ systems. Marburg also rates as one of the most lethal, although the mortality rate varies from outbreak to outbreak, ranging from as low as 24% of infected people in one case up to 90% mortality in the outbreak in Angola. People can spread Marburg Virus through blood, other bodily fluids or contaminated objects or surfaces.
None of the Sudan Virus vaccine candidates nor the Marburg Virus vaccine candidates to date has been licensed yet for use in humans.
Scientists at Rocky Mountain Laboratory in Hamilton working on a vaccine (VSV-MARV) against the Marburg Virus are hoping that their latest efforts may lead to on the ground aid for the latest Marburg outbreak recently reported in Equatorial Guinea, Africa.
African fruit bats are the natural host of Marburg Virus. This is the first outbreak of Marburg Virus disease in Equatorial Guinea. As of last week, nine deaths have been confirmed, while 16 suspected patients are in quarantine. Health officials are also monitoring 15 asymptomatic close contacts of infected people.
The disease also was found in other new parts of Africa in the past two years – Guinea in 2021 and Ghana in 2022 (see map for entire history).

According to Dr. Andrea Marzi, chief of the Immunobiology and Molecular Virology Unit in the National Institute of Allergy and Infectious Diseases (NIAID) Laboratory of Virology at RML in Hamilton, the World Health Organization hopes to test an experimental Marburg vaccine in Equatorial Guinea and convened an urgent meeting last week to evaluate several possible vaccine candidates that could be administered during the outbreak. The meeting brought together a consortium of vaccine developers, researchers and government officials.
Dr. Marzi said that vaccine developers at the meeting wanted to know how many doses of the vaccine were ready for clinical trials and how many could be sent to Equatorial Guinea.
“The answer is we have 300 doses available, if push comes to shove,” said Dr. Marzi. “That’s not a lot.”
But what if they could turn 300 doses into 3,000 by showing that the vaccine dose proven to be effective at preventing the disease in animal trials could be lowered and shown to be just as effective?
“If we could show that a reduced dose, say one reduced by ten times, was just as effective as the proven dose, then we have turned 300 doses into 3,000,” she said.
That could make a big difference. Or just imagine if the vaccine was still effective when the dose is reduced by up to 10,000 times!
Dr. Marzi and her team of scientists at RML designed a study to test the effectiveness of lower doses and how quickly the immune response would take effect at the low doses.
The new study, published in eBioMedicine (Volume 89, 104463, March 202, Kyle O’Donnell, et al )1 [https://doi.org/10.1016/j.ebiom.2023.104463 ] shows that the vaccine (VSV-MARV) provides full protection in cynomolgus macaques at doses up to 10,000 times less potent than originally tested.
The fast-acting potential of VSV-MARV has previously been demonstrated in nonhuman primates. A dose of 1 × 107 PFU resulted in 100% protective efficacy when administered 28, 14, and 7 days prior to lethal MARV challenge. When VSV-MARV is administered three days prior to challenge 75% of the animals survived lethal challenge.
This most recent study demonstrated that lowering the vaccination dose to 1 × 105 or 1 × 103 PFU 14 days prior to challenge retains uniform protective efficacy. When reducing the time between 1 × 103 PFU vaccination and challenge to 7 days, 100% protective efficacy was retained. The data shows that a strong multifunctional humoral response is elicited after vaccination, with fast-acting contributions from innate cellular response and a supportive role of the T cell response.
“We have demonstrated that the VSV-MARV remains fast-acting even at a lower dose highlighting the potential to extend the number of doses from one vial of the limited GMP-manufactured supply,” it states in the study. “Our data supports the administration of a single low-dose to achieve more doses per vial of vaccine in an emergency outbreak situation and decrease the chances of vaccine-induced adverse events. This data also adds to the existing body of evidence that VSV-MARV is a fast-acting vaccine suitable to be administered during outbreaks and supports its further clinical development.”
RML is working with vaccine producer Public Health Vaccines to help move the vaccine through the licensing process. They are one of three companies, including Janssen Pharmaceuticals and Sabin Vaccine Institute, producing five different vaccine candidates for possible use for testing in the current outbreak. The vaccines from Janssen and Sabin have already gone through phase 1 clinical trials. Public Health Vaccines’ shot was recently shown to protect against the virus in animal trials, and the Food and Drug Administration has cleared it for human testing.
“Overall, this data highlights VSV-MARV as a viable and fast-acting MARV vaccine candidate suitable for deployment in emergency outbreak situations and supports its clinical development,” said Dr. Marzi.
Will any vaccine reach Equatorial Guinea in time to be used in the most recent outbreak?
“That is the hope, but we will have to see. Things are not moving fast, at least not as fast as we would want them to,” said Dr. Marzi.